How Can You Identify a Substance if the Mass and the Volume Are Known?
2.4: Density and its Applications
- Folio ID
- 3552
The density of an object is 1 of its most of import and easily-measured concrete properties. Densities are widely used to place pure substances and to characterize and judge the composition of many kinds of mixtures. The purpose of this lesson is to evidence how densities are defined, measured, and utilized, and to make certain you understand the closely-related concepts of buoyancy and specific gravity, and the roles they play in our lives and the environment.
What is Density?
About of us accept long understood that "oil is lighter than h2o", or that iron is "heavier" than sugar. But in making such statements, nosotros are implicitly comparing equal volumes of these substances: later all, we know that a cup of sugar will counterbalance more than a single ordinary steel boom. Mass and book are measures of the quantity of a substance, and as such are defined equally extensive backdrop of matter. The ratio of two extensive backdrop is e'er an intensive property — one that characterizes a particular kind of matter, independently of its size or mass. Information technology is this ratio, (mass ÷ book), that we are concerned with in this Module.
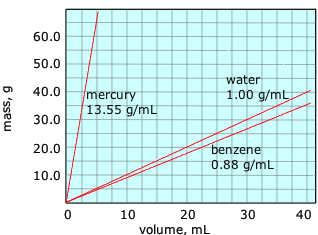
These plots show how the masses of 3 liquids vary with their volumes. Notice that
- the plots all have the aforementioned origin of (0,0): if the mass is zero, so is the volume;
- the plots are all directly lines, which signify direct proportionality.
The merely difference betwixt these plots is their slopes. Denoting mass and volume past \(k\) and \(Five\) respectively, nosotros tin can write the equation of each line as \(thou = \rho V\), where the slope \(\rho\) (Greek lower-example rho) is the proportionality constant that relates mass to volume. This quantity \(\rho\) is known every bit the density, which is usually divers equally the mass per unit volume:
\[\rho = \dfrac{g}{V}.\]
The volume units milliliter (mL) and cubic centimeter (cm3) are identical and are ordinarily used interchangeably.
The general significant of density is the amount of anything per unit of measurement volume. What we conventionally call the "density" is more precisely known as the "mass density".
Density can be expressed in any combination of mass and volume units; the most commonly seen units are grams per mL (grand mL–1, m cm–3), or kilograms per liter.
1 kg m–iii = x–3 g L–1 = 62.4 lb ft–3
Densities of Common Substances
The range of densities encountered in the world spans a remarkably wide range, from substantially zero in outer space to the unimaginably huge values found in stellar bodies. These very high densities represent the ultimate limits of how much mass can exist packed into a given volume. The following nautical chart will requite you some feeling for the values of density constitute in nature generally (peak), in mutual solids (middle), and in gases and liquids (bottom). Delight note that in order to describe reasonably wide ranges of values in limited space, the density scales are logarithmic ; thus null on these scales corresponds to the density of water (100 = ane g cm–3). Densities listed for ordinary substances (including gases) are mostly those at around twenty° C.
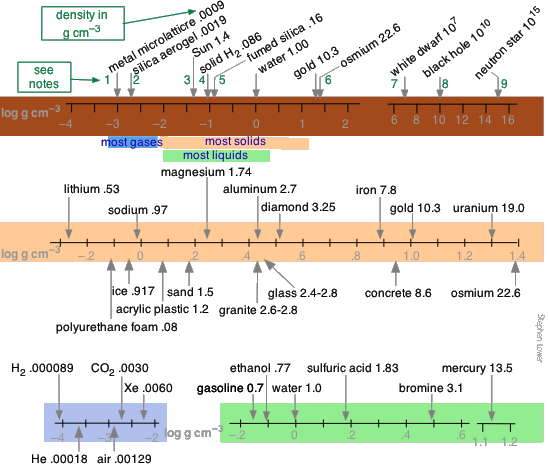
Solids, liquids and gases
In general, gases have the lowest densities, simply these densities are highly dependent on the pressure and temperature which must always be specified. To the extent that a gas exhibits ideal behavior (low pressure, high temperature), the density of a gas is directly proportional to the masses of its component atoms, and thus to its molecular weight. Measurement of the density of a gas is a simple experimental way of estimating its molecular weight.

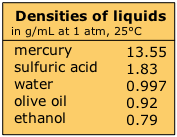
Liquids embrace an intermediate range of densities. Mercury, existence a liquid metallic, is something of an outlier. Liquid densities are largely independent of pressure, but they are somewhat temperature-sensitive.
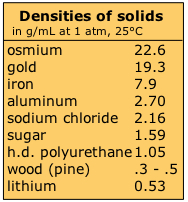
The density range of solids is quite wide. Metals, whose atoms pack together quite compactly, have the highest densities, although that of lithium, the highest metallic element, is quite low. Blended materials such equally forest and loftier-density polyurethane foam contain void spaces which reduce the average density.
How the temperature affects density
All substances tend to expand as they are heated, causing the same mass to occupy a greater volume, and thus lowering the density. For nigh solids, this expansion is relatively small, merely it is far from negligible; for liquids, it is greater. The volumes of gases, as you may already know, are highly temperature-sensitive, and so, of course, are their densities.
What is the cause of thermal expansion? As molecules learn thermal free energy, they move about more vigorously. In condensed phases (liquids and solids), this motion has the character of an irregular kind of bumping or jostling that causes the boilerplate distances between the molecules to increase, thus leading to increased volume and smaller density.
Applications of density
Lava Lamps
Known more generically as "fluid motion lamps", these devices became popular in the 1970's and provide a overnice, if somewhat mesmerizing analogy of density and buoyancy in action as the blobs of oozing goo move upwardly and downward in ever-irresolute shapes. These lamps consist of a container of h2o in which is placed a colored organic oily liquid that does not mix with water, thus constituting a second phase. The composition of the oil phase is such that its density is slightly greater than that of water at room temperature, so it ordinarily resides at the lesser of the container. When the lamp is turned on, a oestrus source (usually an incandescent light seedling) concealed in the base of the container heats the oil stage. This reduces its density to a value below that of the water, causing blobs of oil to rise to the meridian of the container. Beingness now far removed from the heat source, the blobs absurd down and sink back to the bottom, where they repeat the cycle.
francislearallings.blogspot.com
Source: https://chem.libretexts.org/Courses/Palomar_College/PC:_CHEM100_-_Fundamentals_of_Chemistry/03:_Matter_and_Energy/2.4:_Density_and_its_Applications
0 Response to "How Can You Identify a Substance if the Mass and the Volume Are Known?"
Post a Comment